Isotretinoin (formerly known as Accutane) is a highly effective oral retinoid used to treat severe and cystic acne. This vitamin A derivative works by reducing acne inflammation and significantly decreasing sebum (oil) production. Because excess oil and inflammation are major causes of acne, isotretinoin targets the condition at its root.
If you are exploring other options first, visit our Acne Treatment in St. Louis page to learn more about available therapies.
How Isotretinoin Became a Gold Standard Treatment
When isotretinoin was first introduced in 1982, it was prescribed primarily to teenagers with severe flaring acne. Physicians quickly discovered that it worked extremely well — and, importantly, patients did not need to stay on the medication permanently. A typical 6–8 month course often led to long-term or permanent acne clearance.
However, early reports raised concerns about depression and suicidal thoughts. As a result, the U.S. Food and Drug Administration placed a black box warning on isotretinoin. Since then, multiple large-scale studies have evaluated these concerns. Research has not shown a proven causal link between isotretinoin and depression. In fact, many patients report improved mood as their acne clears and their confidence increases.
Today, isotretinoin continues to be safely prescribed by board-certified dermatologists across the United States.
What Is the iPLEDGE Program?
Although isotretinoin is safe for most patients under supervision, it can cause severe birth defects if taken during pregnancy. For this reason, strict regulations are enforced through the iPLEDGE program.
The iPLEDGE system exists solely to prevent pregnancy during treatment with isotretinoin.
iPLEDGE Requirements:
- Female patients of childbearing potential must use two forms of birth control and complete monthly pregnancy tests before receiving refills.
- Male patients must also register in the iPLEDGE system.
- Prescriptions are limited to a 30-day supply, and monthly physician visits are required for renewal.
You can learn more about medication safety monitoring through the National Institutes of Health.
What to Expect During Isotretinoin Treatment
Most patients complete a 6–8 month course of isotretinoin and do not require additional acne therapy afterward. While results are often long-lasting, side effects can occur.
Common side effects include:
- Dry lips
- Dry nose
- Dry skin
Occasionally, isotretinoin may raise triglyceride levels, so your dermatologist may monitor blood work during treatment.
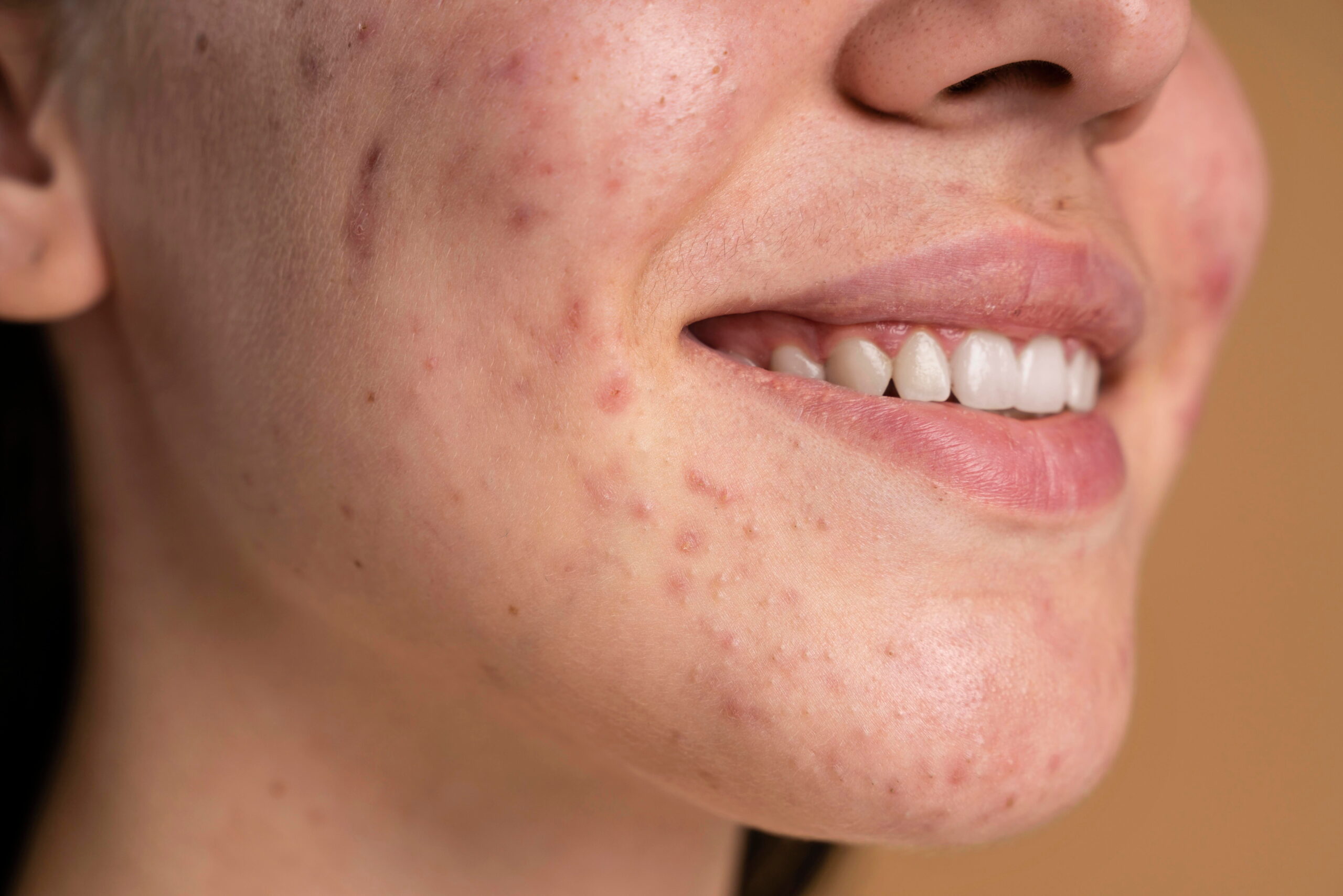
Is Isotretinoin Right for You?
If you have severe, scarring, or treatment-resistant acne, isotretinoin may offer the long-term solution you need. Early treatment can prevent permanent scarring and reduce the emotional burden of chronic acne.
To learn whether isotretinoin is appropriate for your skin, click the “Request Appointment” button and schedule your consultation today.